Abstract
High-risk myelodysplastic syndrome (HR-MDS) has a poor outcome, and not many patients can benefit from the standard of care with azacitidine (AZA) monotherapy. The main reasons for the poor prognosis include transformation to acute myeloblastic leukemia (AML), adverse events, such as infection, and intolerance to therapy. The transformation to AML is frequently associated with features of cytogenetic abnormalities, while the patients' conditions, like nutritional status and frailty, influence the emergence of infectious complications and therapeutic intolerance. In addition, the importance of the nutritional assessment has been reported in other cancers, and albumin, total cholesterol (T-Cho), and body weight have been often used as nutritional parameters. However, the conventional prognostic indices for MDS, such as the International Prognostic Scoring System (IPSS-R) and Revised IPSS (IPSS-R), do not include those factors. In this study, we tried to develop a novel prognostic tool by reassessing the prognostic values of pre-existing prognostic factors with nutritional assessment in patients with MDS treated by AZA.
One hundred six patients who started AZA monotherapy between 2011 January and 2021 December at four independent institutes (University Hospital Kyoto Prefectural University of medicine, Fukuchiyama City Hospital, Aiseikai Yamashina Hospital, and Kyoto Kuramaguchi Medical Center), belonging to the Kyoto Clinical Hematology Study Group were retrospectively analyzed by collecting clinical and survival data using case report forms. Data collected include factors involved in IPSS-R score (absolute neutrophil count (ANC), hemoglobin level (Hb), platelets (Plt), rate of bone marrow (BM) blasts and karyotype), serum levels of albumin and T-Cho, age, gender, and body mass index (BMI). Overall survival (OS) was defined as the time from the initiation date of AZA treatment to death from any cause or the date of the last follow-up, and was estimated using the Kaplan Meier method. Females were 26 (24.5%) and the median age was 73 years (31-92). According to IPSS-R, 54 (50.9%) patients were classified as very high, 31 (29.2%) as high, 18 (17.0%) as intermediate, and 3 (2.8%) as low. With the median follow-up period of 8.7 months (0.6-55.0), the median OS of the entire cohort was 10.2 months (95% confidence interval (CI), 8.8-12.8). By the univariate analysis by Cox proportional hazard analyses for OS, we identified T-Cho, Hb, albumin, and cytogenetic risk defined by IPSS-R as poor prognostic factors with the cutoff p-value of 0.1. By the multivariate analysis using these factors as variables, T-Cho and CR were found to be significantly associated with poor prognosis in our cohort (p-value; 0.013 and 0.006, respectively).
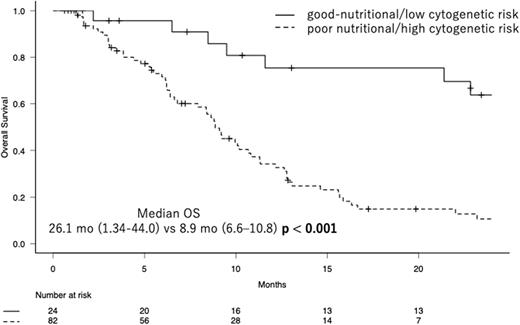
Accordingly, we divided patients into two groups, i.e., a group of patients with good nutritional status and low cytogenetic risk and the other group of patients with poor nutritional status and/or high cytogenetic risk, and we here designated this classification as the nutritional and cytogenetic classification (NCC). With this classification, T-Cho< 150 mg/dL was considered to be indicative of poor nutrition, while the high cytogenetic risk was defined as very poor in IPSS-R. In NCC, 24 patients (22.6%) were classified as a good-nutritional/low cytogenetic risk group, while others were as a poor nutritional/high cytogenetic risk group. As the result, the median OS of the former was 26.1 months (95% CI, 21.34-44.0), while that of the latter was 8.9 months (95% CI, 6.6-10.8) (p <0.001, log-rank test). In addition, the former group was significantly associated with higher Hb. The C-indices of IPSS-R and NCC were 0.595 (standard error (SE); 0.033) and 0.611 (SE; 0.027), respectively, showing a more favorable risk discrimination ability of NCC than IPSS-R in our cohort.
In conclusion, we here propose a new prognostic model, designated as NCC, for patients with MDS treated by AZA monotherapy, which, incorporates the serum level of T-Cho as a prognostic factor in addition to the cytogenetic risk, of which C-index was higher than the conventional IPSS-R.
Disclosures
Kuroda:Chugai Phamaceutical: Research Funding, Speakers Bureau; Japan Blood Products Organization: Research Funding; Daiichi Sankyo: Research Funding, Speakers Bureau; Ono Pharmaceutical: Research Funding, Speakers Bureau; Sanofi: Research Funding, Speakers Bureau; Eisai: Research Funding, Speakers Bureau; Taiho Phamaceutical: Research Funding; Sumitomo Pharmaceutical: Research Funding, Speakers Bureau; Otsuka Pharmaceutical: Research Funding, Speakers Bureau; Asahikasei: Research Funding, Speakers Bureau; Takeda Pharmaceutical: Research Funding, Speakers Bureau; Nippon Shinyaku: Speakers Bureau; Janssen Pharmaceutical: Speakers Bureau; CLS Behring: Research Funding; Bristol Myers Squibb: Research Funding, Speakers Bureau; Abbvie: Research Funding, Speakers Bureau; Fujimoto Pharmaceutical: Research Funding, Speakers Bureau; Sysmex cooporation: Research Funding; Kyowa Kirin: Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.